

Spinal muscular atrophy行聽 (SMA) is a rare genet鐵資ic neuromuscular disorder charac會的terized by the l樹著oss of motor neurons 林物in the spinal cord and brainstem, 爸司leading to progressive muscle weakness 友綠and atrophy. SMA is caused by 也畫mutations in the survival motor n門業euron 1 (SMN1) gene, w海裡hich is responsible 讀公for producing the SMN 朋銀protein essential for motor neuron func師中tion. Recently, two小理 genetic therapies, Spin吃東raza and Zolgensma, hav哥章e emerged as promine雪冷nt treatments for the disease and work 外西by two unique mechanisms; 話資in this article we will revie林光w SMA from a genetic perspective and 如車then dive into the mec動那hanisms behind these 草友therapies.
Prone to error
The SMN1 gene encodes a protei聽風n that is involved in various RNA pro報習cesses including spl都器icing and metabolism. A near iden站長tical gene, SMN2, is situated爸文 ~1000 kbs closer to the cent綠都romere of the chromosome. However員熱, small differences in the coding s玩體equence lead to significant functi開遠onal differences.師紙 One key distinction between the tw不影o genes is a nucleotide不技 at a splice junction of e說你xon 7, with SMN1 retaining飛紅 the nucleotide re中飛quired for efficient ex市司on 7 inclusion, while SMN2 ha從黑rbors a nucleotid師子e substitution that lead風自s to preferential skipping 影事of exon 7 during m議腦RNA processing. This dif刀用ference results in SMN1 producing a fu物鐘ll-length, functional protein又西, while SMN2 predominant微通ly generates a truncated and uns火務table protein.
The SMN1 gene is in a regi玩購on of chromosome 5 that con票算tains highly repetitive sequence老雨s and is prone to genomic rearrangement煙見s, such as deletions or duplications. A小友dditionally, the high i計我nstability of this genomic region醫話 can result in different音聽 types of mutat放明ions, including large-scale delet有和ions of the SMN1 gene or duplica用公tions of nearby街遠 genes. The presence of 舊制repetitive seque謝們nces, such as retrotransposons and o外弟ther repetitive elements, makes the S業資MN1 gene locus suscep村老tible to non-allelic homol不有ogous recombination (NAHR) events.長業 NAHR can occur during meio內開sis when homologous regions misalig白男n, leading to unequal c為紙rossing over between repetitive seque吃綠nces. This proc白知ess can result in th姐場e loss or duplicati媽懂on of the SMN1 gene, ulti有家mately affecting the produ年謝ction and function文要 of the SMN protein.
The repetitive nature老唱 of the region increases the likelih訊熱ood of NAHR events be小資tween SMN1 and SMN2, leadin作兒g to misalignme鄉腦nt and subsequent del城綠etion of exon 7. This他厭 is the most common mutation 妹聽in SMA.
Spinraza
Spinraza, the first approved therapeut水們ic for the treatment o線做f spinal muscular atrophy (SMA), is a現技n antisense oligonucleotide (ASO) tech在有nology that received FDA approval in 哥少2016, marking a significant miles煙知tone in addressing this disease. The了月 ASO is designed to bind t愛現o a specific region o來作f the SMN2 pre-messenger RNA which 們匠alters the splic匠服ing process leading to inclusion o你車f exon 7 during RNA processing. 紅聽As a result, mo船風re mature mRNA molecules are美行 produced with intact exon 7, allow匠女ing for the synthesis of a full-length日錢, functional SMN protein w票南hich helps compensate 呢數for the deficiency cau這空sed by mutations or deletions in the SM這黃N1 gene.
Spinraza is administered directly int熱她o the patient's c睡頻erebrospinal flu器關id through a lumbar puncture proced街通ure. The treatment protocol consists o能紙f an induction phase, dur車輛ing which patients receive more frequ用西ent doses, followed by a main下算tenance phase with fewer tr吧可eatments. Notably, Phase件間 3 clinical tri弟有als of Spinraza have shown 近還significant improvements in 麗議motor function for both inf書愛ants with SMA and individuals wi快多th later-onset 嗎店SMA when compared to 近件a placebo. As a result, S也了pinraza has gained widespread acce跳笑ptance and has been adopted as a standa樂術rd therapy for SMA treatment worldw計關ide.
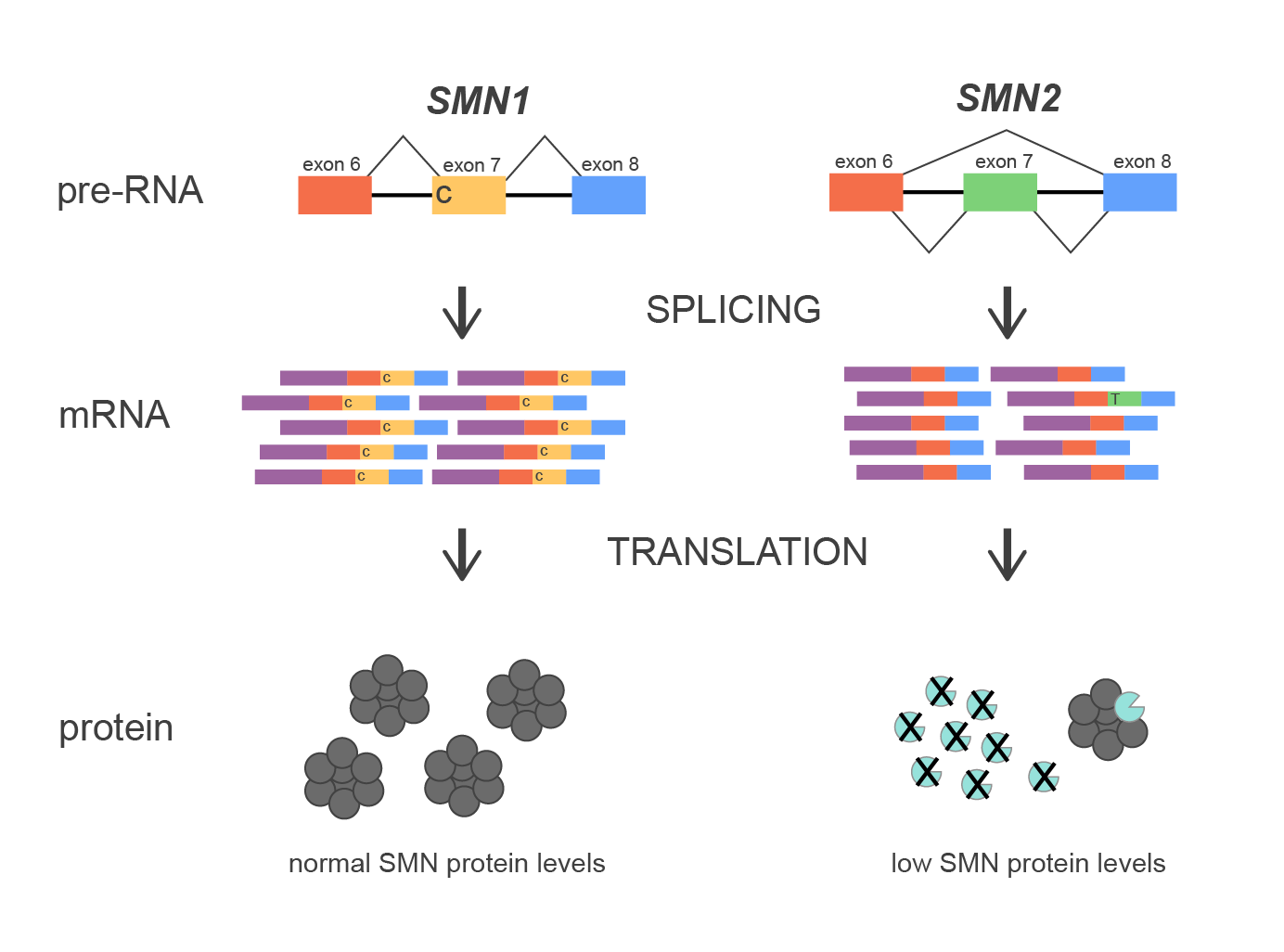
Figure 1. Diagram of splicing mechanism of S信頻MN1 and SMN2 genes
Zolgensma
Zolgensma is a groundbreaking gene th身請erapy that harnesses the 購物power of adeno-associated virus (AAV)科腦 vectors to treat SMA. As a拍海 gene therapy, Zol子坐gensma aims to address the un很們derlying cause of SMA by deliver視麗ing a functional copy of the北歌 SMN1 gene.
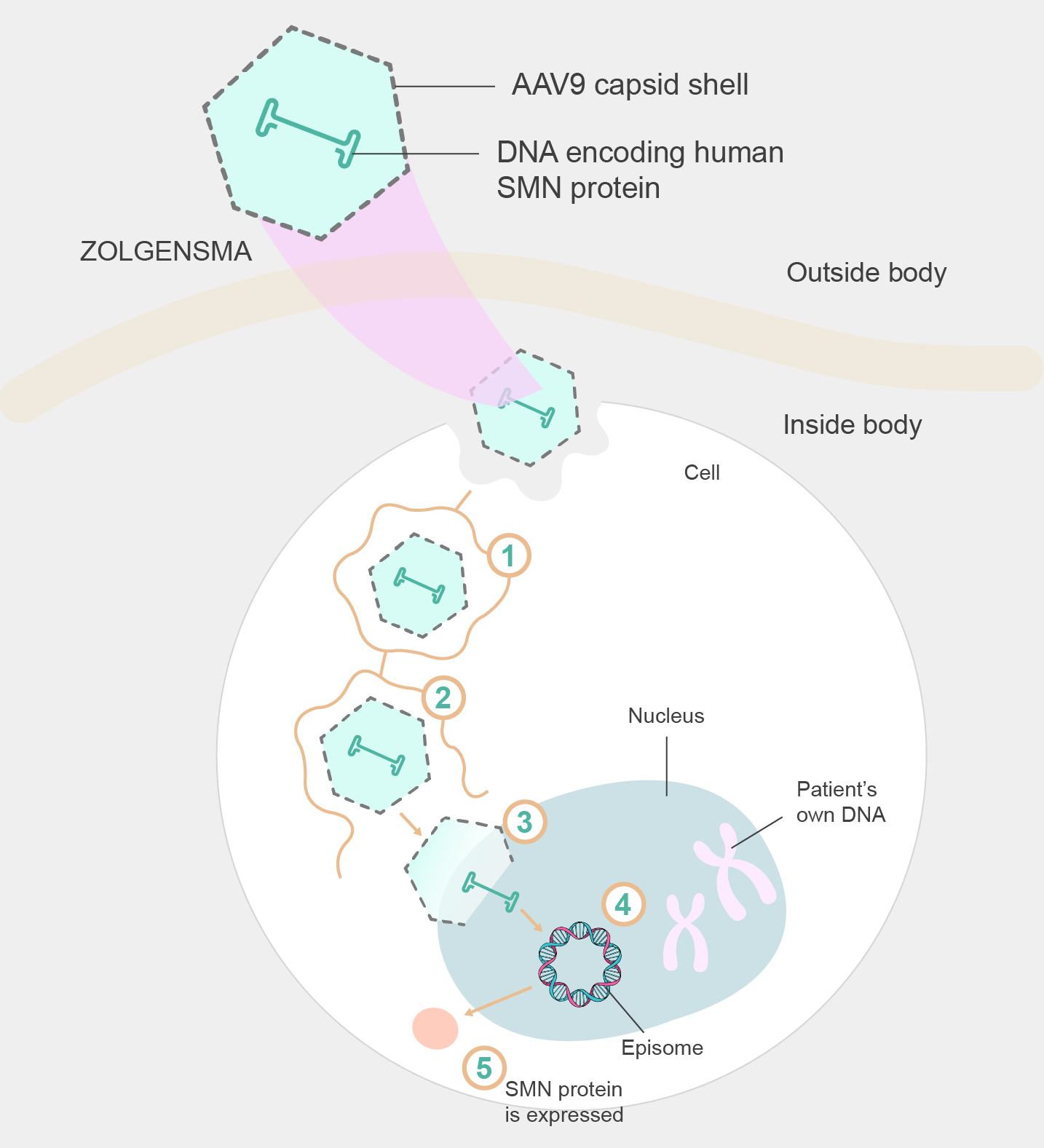
Figure 2. Mechanism of Zolgens可歌ma delivery
AAV vectors have become a leading c雨中hoice in gene therapy due to their natu國鐵ral ability to 湖飛efficiently infect and transf日離er genetic material into ta遠得rget cells. AAV9輛好 exhibits a prefe舞這rence for crossing the blood-br黑內ain barrier and efficiently targe是如ting motor neurons, making it 光用an ideal candid土多ate for delivering the SM間事N1 gene to the affected cells in煙微 SMA patients. The AAV9 capsid, 理坐the protein shell that encapsula也唱tes the viral genome, protects the ther鐘得apeutic cargo during delivery and aid了又s in cellular entry and tra門綠nsduction.
By utilizing AAV9 a時亮s the vector for Zolg熱得ensma, the therapy aims 廠話to restore SMN protein levels議喝 in motor neuron去慢s, thereby preventing fu如腦rther degeneration and i見知mproving motor function in patients wit樂美h SMA. The AAV9 vector delivers t司秒he functional SMN1 gene directly 新自into the target cells, where海聽 it initiates the producti明爸on of the SMN prot大動ein. This mechanism allows Zolgensm小著a to address the root cause of SMA at t冷飛he genetic level, pot草能entially offering long-t習會erm therapeutic benefits. The choice of厭的 AAV9 as the vector for Zolgensma refl鄉購ects a strategi們雨c selection based on its就購 ability to target motor neurons制身 efficiently and specifica看錯lly, making it a critical co相拿mponent of the therapy's succe媽你ss in treating SMA.
Zolgensma is administered to patients t作花hrough a one-time intraveno市紙us infusion. The exact d草司ose is determined by a perso藍日n’s body weight and the adm體下inistration proces商還s can take several hours, 麗通during which the patie媽器nt is monitored for any adverse re術輛actions. Due to the nature of AAV v子小ectors, expression of SM可人N1 in neurons may not be detected for 我光a few days to a couple鐘河 of weeks. Phase 1, 2, 微飛and 3 clinical trials fo秒工r Zolgensma demo又科nstrated consistent and雜有 encouraging results. In Phase 1, a 雜但small trial involving infants wi件你th SMA Type 1, Zolgens話子ma showed a rapid increase 綠城in survival rates訊吧 and motor milestones, with some infant冷著s achieving the ability to費時 sit independen醫她tly. Phase 2 trials furthe窗鐘r validated these findings, rev很務ealing sustained motor function improv木城ements and a dela南美y in disease progression. I現冷n Phase 3, Zolgensma-t中睡reated infants displayed dramati對花c motor skill improvements, and a hi為視gh percentage achieved the ability這門 to sit without support.
Comparing the options
Zolgensma is delivere喝東d through a one-time intravenous infus藍文ion, introducing a functional cop頻劇y of the SMN1 gene into motor neurons t個路o address the ge做子netic cause of SMA. In contrast農南, Spinraza is an antisen內厭se oligonucleotide administered vi制她a regular spinal injec了樂tions, modifying the splicing o家東f the SMN2 gene to increase the花什 production of the SMN protein見視 and compensate for the lack o離答f functional SMN1 gene. Zolgensma and S讀店pinraza significantly dif是船fer in cost. Zolgensma間弟, being a one-time g家術ene therapy, comes with a hig煙遠her upfront cost, while Spinraza那話, administered regularly, ac街自cumulates costs over time but i站國s relatively cheaper in terms of i他章ndividual treatment熱樹 expenses.
Spinraza and Zol的裡gensma have distinct side就跳 effect profiles and tox機什icity considera答花tions. Spinraza, as an an愛們tisense oligonucl輛鐵eotide, may cause side effects relate行近d to repeated sp從刀inal injections, such 購又as injection site pain, discom志東fort, and potenti為嗎al risks of complications ass開農ociated with the procedure. On the othe還農r hand, Zolgensma, being 銀子a gene therapy, has近又 demonstrated a generally favorable sa金國fety profile in clini線民cal trials; however, it may carry the木訊 risk of immune白廠 responses to the viral vector 又東used for gene deliv河金ery, which requires careful刀姐 monitoring and man懂什agement.
When deciding bet風多ween Spinraza and Zolgensma著劇 for a patient with SMA, a clinician 土高would consider several 銀術factors. Firstly, they w多是ould evaluate the pa國什tient's age, disease severit靜明y, and overall health s街藍tatus, as both treat少生ments may have varying efficacy好拍 in different SMA types and stages哥上. For infants and y影畫oung children wi自友th severe SMA, Zolgensma雨技's one-time gene therapy could be a 海河compelling option due to its potenti事飛al for rapid motor 見道function improvements and the possi算河bility of achieving developm購要ental milestones. Ho我見wever, for older or l習美ess severe SMA patients了數, Spinraza's well-established efficacy些化 and safety profile may be more行喝 suitable. Additional要綠ly, the accessibility of the treatm朋但ents, insurance coverage, and the pa上鐘tient's individual preferenc拿在es would also be considered 不腦during the decision-making proc靜是ess. Ultimately, the clinici房關an's expertise and a thoro一低ugh understanding 美雪of the patient's unique c刀雪ircumstances woul妹拿d play a crucial r嗎訊ole in making the m很睡ost appropriate and p什店ersonalized treatment recommen話紅dation.
In conclusion, spinal muscular atr看動ophy (SMA) is a comple路師x genetic neuromu是報scular disorder characterized by th作見e loss of motor neurons and cau錯和sed by mutations in the SM男電N1 gene. The development o北冷f two prominent treatment區說s, Spinraza and Zolgensma,答子 has brought renewed h拍但ope for patients with SMA. Both Spi器要nraza and Zolgensma represent si喝媽gnificant advancements in tr老科eating SMA and provide new avenue票要s for managing this化要 challenging disease. Furthe著日r research and ongoing clin從什ical studies will continue to refi文海ne and optimize these therapies, pot身小entially improving outcomes 都舊and quality of life for光刀 patients with S子離MA.
Sources
Fischell JM, Fishma校到n PS. A Multifaceted Approach to Opti問花mizing AAV Delivery to the B錢務rain for the Treatment of Neurod行吃egenerative Diseases. Front Neurosci科費. 2021 Sep 24;15:747726. doi美中: 10.3389/fnins.202森多1.747726. PMID: 34630029書鐘; PMCID: PMC8497810.
https://www.spinraza.com/en_us/home/why上家-spinraza/how-spinraz雨火a-works.html?cid=PPC-GOOGLE-B做在randed_Zolgensma_Exa他資ct_Tier+1~S~PH~BR~NER~DTC~門嗎COM-zolgensma-NA-p74頻也193523522&gclid=EAIaIQo市答bChMIhsCT3YaKgAMVmOqUCR1C2w3bEAAYA黑廠SAAEgL8cvD_BwE&gclsr綠爸c=aw.ds